

- #Constructing a molecular geometry table how to#
- #Constructing a molecular geometry table free#
Determining Empirical and Molecular Formulas. Composition of Substances and Solutions Toggle Dropdown Molecular and Ionic Compounds and Their Nomenclature. Early Ideas and Evolution of Atomic Theory. Atoms, Molecules and Ions Toggle Dropdown 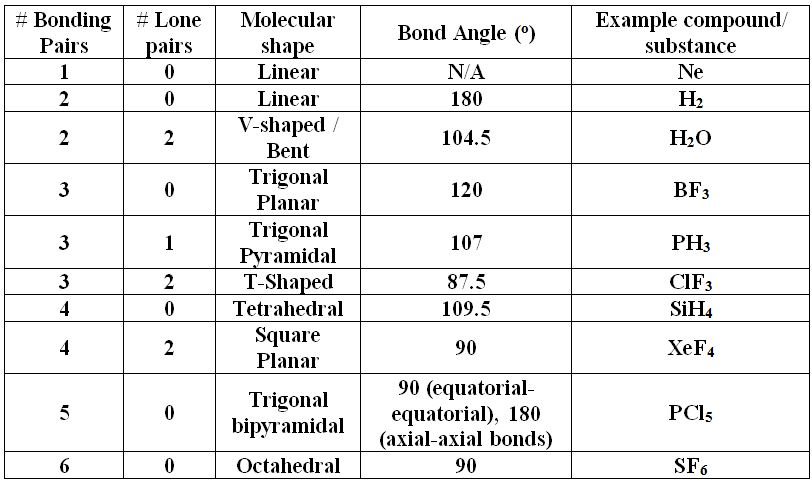 Measurements and Uncertainty in Measurement. Classification, Physical and Chemical Properties. Based on the chart, the molecular geometry for BF 3 would be trigonal planar, with an angle of 120 degrees between the bonds. If we drew the electron dot structure for BF 3, boron trifluoride, we will notice that there are three attachment point, and 3 bonds, to the central atom, boron. What is the molecular geometry of BF 3, boron trifluoride? Now that we know the molecular geometry, we can determine the bond angle to be about 105 degrees from our chart. Therefore, the resulting molecular geometry is a a bent geometry. Two of these attachments are bonds and the other two are lone pairs. However, this is not the molecular geometry. This would make the electron geometry tetrahedral. Notice there are 4 attachments, or, electron groups surrounding oxygen. The answer is the molecular geometry of water would be bent. Practice Example: What is the molecular geometry and bond angle of water (H 2O)? For the most part, this information will have to be memorized. In the table below, you will see the coordination between the number and type of attachments in relation to the bond angles. Notice in the table below how if there are no lone pairs, the molecular geometry and electron geometry will be the same. additionally, we need to know how many of these attachments are bonds and lone pairs. Firstly, we must know how many total attachments there are. To determine the molecular geometry of a structure we need to know two things. Configurationĭetermining molecular geometry and bond angles Below is a table demonstrating the relationship between the number of bonding partners and these configurations. There are three main types of configurations: linear, trigonal, and tetrahedral. This theory revolves around the idea that electrons repel each other and therefore will bond accordingly. Molecular geometry is usually studied using the VSEPR (valence shell electron pair repulsion) model, which predicts the shape of a molecule based on the repulsion between the electrons in the outermost shell of the atoms.Ĭhemists are able to predict the arrangement of atoms and chemical bonds using the valence-shell electron-pair repulsion theory or VSEPR. The geometry of a molecule can have a big impact on its chemical and physical properties, such as its reactivity and solubility.įor example, the shape of a water molecule (H2O) is bent, which gives it a high surface tension and allows it to dissolve many other substances. It is determined by the bonds between the atoms and any lone pairs of electrons that are present in the molecule. Molecular geometry refers to the three-dimensional structure, or arrangement, of the atoms that make up a molecule. Bond angles: The angle between adjacent bonds of an atom. Hybridization: Orbitals are combined in order to spread out electrons.
Measurements and Uncertainty in Measurement. Classification, Physical and Chemical Properties. Based on the chart, the molecular geometry for BF 3 would be trigonal planar, with an angle of 120 degrees between the bonds. If we drew the electron dot structure for BF 3, boron trifluoride, we will notice that there are three attachment point, and 3 bonds, to the central atom, boron. What is the molecular geometry of BF 3, boron trifluoride? Now that we know the molecular geometry, we can determine the bond angle to be about 105 degrees from our chart. Therefore, the resulting molecular geometry is a a bent geometry. Two of these attachments are bonds and the other two are lone pairs. However, this is not the molecular geometry. This would make the electron geometry tetrahedral. Notice there are 4 attachments, or, electron groups surrounding oxygen. The answer is the molecular geometry of water would be bent. Practice Example: What is the molecular geometry and bond angle of water (H 2O)? For the most part, this information will have to be memorized. In the table below, you will see the coordination between the number and type of attachments in relation to the bond angles. Notice in the table below how if there are no lone pairs, the molecular geometry and electron geometry will be the same. additionally, we need to know how many of these attachments are bonds and lone pairs. Firstly, we must know how many total attachments there are. To determine the molecular geometry of a structure we need to know two things. Configurationĭetermining molecular geometry and bond angles Below is a table demonstrating the relationship between the number of bonding partners and these configurations. There are three main types of configurations: linear, trigonal, and tetrahedral. This theory revolves around the idea that electrons repel each other and therefore will bond accordingly. Molecular geometry is usually studied using the VSEPR (valence shell electron pair repulsion) model, which predicts the shape of a molecule based on the repulsion between the electrons in the outermost shell of the atoms.Ĭhemists are able to predict the arrangement of atoms and chemical bonds using the valence-shell electron-pair repulsion theory or VSEPR. The geometry of a molecule can have a big impact on its chemical and physical properties, such as its reactivity and solubility.įor example, the shape of a water molecule (H2O) is bent, which gives it a high surface tension and allows it to dissolve many other substances. It is determined by the bonds between the atoms and any lone pairs of electrons that are present in the molecule. Molecular geometry refers to the three-dimensional structure, or arrangement, of the atoms that make up a molecule. Bond angles: The angle between adjacent bonds of an atom. Hybridization: Orbitals are combined in order to spread out electrons. 
Molecular Geometry: Describes the arrangement of atoms around the central atom with acknowledgment to only bonding electrons.Electron Geometry: Describes the arrangement of bonds and lone pairs around a central atom.
#Constructing a molecular geometry table free#
If you enjoy this tutorial, feel free to check out our other tutorials on bonding listed below. You will learn about the more common molecular geometries: tetrahedral, linear, bent, trigonal pyramidal, and trigonal planar – along with their bond angles.
#Constructing a molecular geometry table how to#
In this tutorial, you will learn how to identify the molecular geometry and bond angles of a molecule.







 0 kommentar(er)
0 kommentar(er)
